Past News & Events
OPEX Oil & Gas Summit 2019
November 4-6, 2019
ATR’s participated as an associate sponsor in the OPEX oil and gas summit at Norris Conference Center's Houston CityCentre!
Elliott Lander's workshop on Smart Digital Procedures was well attended by executives from companies like Dow, INVISTA, Marathon Petroleum Corporation, and Baker Hughes, among others. It was an extremely engaging and interactive session, to say the least! ATR exhibited at the 2-day expo and showcased the digital procedure lifecycle management solution, SmartProcedures.
Monument Inn Lunch & Learn
September 26, 2019
ATR arranged a fantastic Lunch and Learn event in La Porte, Texas that was well-attended by executives from Dow, LyondellBasell, The Lubrizol Corporation, AmSty, Vopak, ONEOK, Evonik, Kuraray, and others.
This event was focused on digital transformation for enhanced safety, reliability, and operational excellence. It provided some great opportunities to learn about building better human-factored procedures.
The speakers were Mark Staes, Competency Development & Assurance Manager from LyondellBasell, Tim Watson, Director, Environmental, Health and Safety from AmSty, and Elliott Lander, Managing Director from ATR.
NAPTA 2019
September 24-26, 2019
ATR attended NAPTA, the instructor Skill Conference, on the 24th and 25th September 2019 in League City, TX.
ATR Inc has consistently been a leader in providing a learning platform for optimum performance and work guidance solutions to procedure writers focused on human factored procedure writing practices. In close collaboration with Texas A&M University and the TEES Mary Kay O'Connor Process Safety Center-TAMU, ATR Inc is playing a vital role in creating an exciting new phase of digital procedure usage and workforce acceptance.
Visitors learned about the importance of digital human factored procedures and best practices in the digital transformation process as a path to operational excellence and enhancement of operational safety and reliability!
Webinar: Operational Discipline and The Criticality of Smart Digital Procedures
September 5, 2019
The demand for operational excellence continues to grow as regulations tighten, businesses look for effective ways to improve margins, reliability, and safety. Many companies implement operational excellence strategies but can often neglect the most important aspect of their complex and hazardous business - the front line worker.
This exclusive webinar focused on:
- The relationship between conduct of operations, operational discipline and operating procedures
- Why current procedure practices are putting your operations at risk and increasing potential for human error
- Challenges experienced with current procedure practices and how the aging workforce is pushing digital transformation
- How to improve procedure usability and effectiveness using a proven "fit-for-purpose" procedure technology
- Why digital and mobilized procedures will change the "day in the life" of your operations personnel
- How digital procedure data provides critical business intelligence to optimize performance and reliability
Procedure Excellence Writer Workshop
November 7, 2018
We invite you to join us and learn how the industry is improving operational excellence by harnessing human factors research and technology advances to improve procedure quality and usability.
Highlights of our writer workshops include:
- Writing and rewriting complex procedures to incorporate human factoring for various clients
- Applying evidence-based research to improve procedures and procedure programs
- Advising and learning from companies in implementing optimized procedure solutions for human performance, safety, and compliance
- Teaching best writing practices to procedure professionals across several industries
- Utilizing research-driven techniques to improve human performance, safety, and compliance
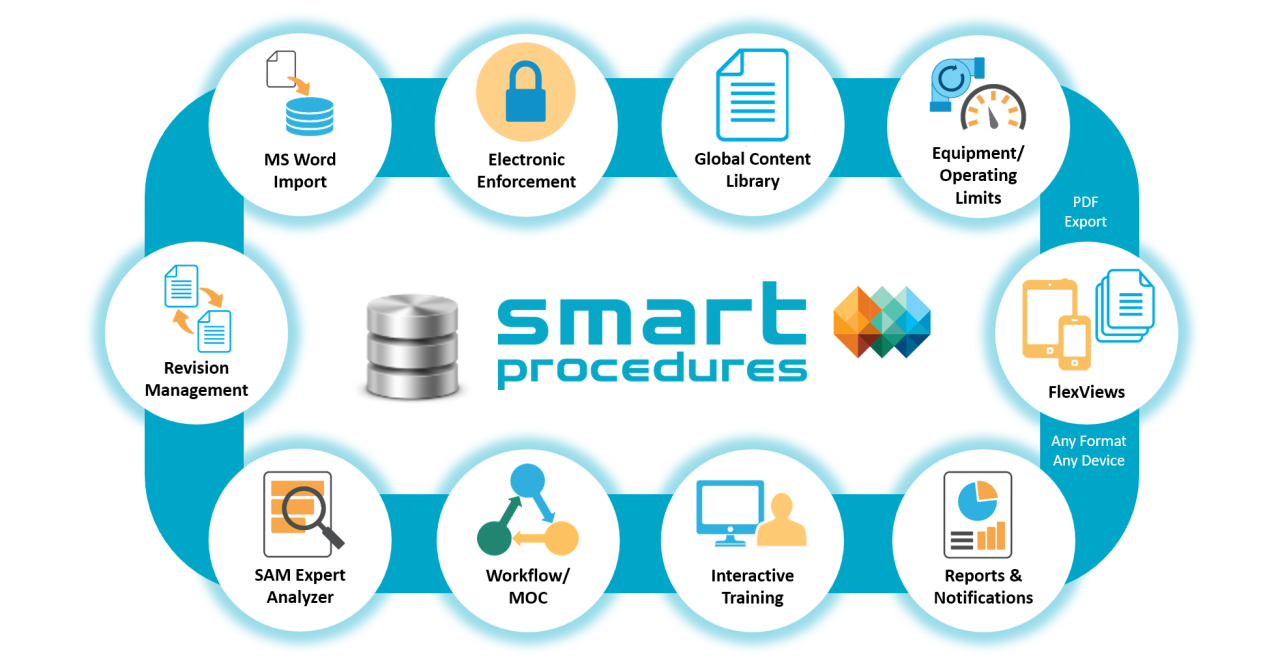
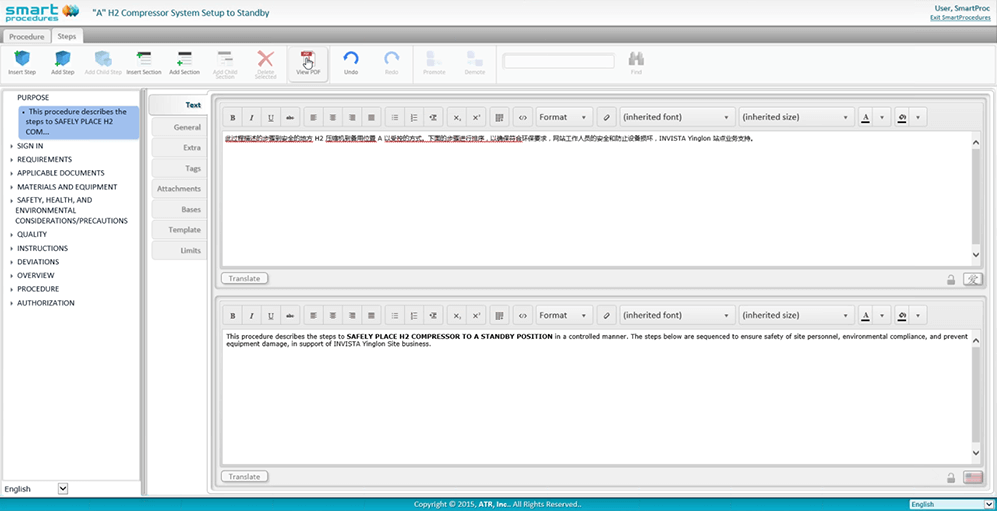
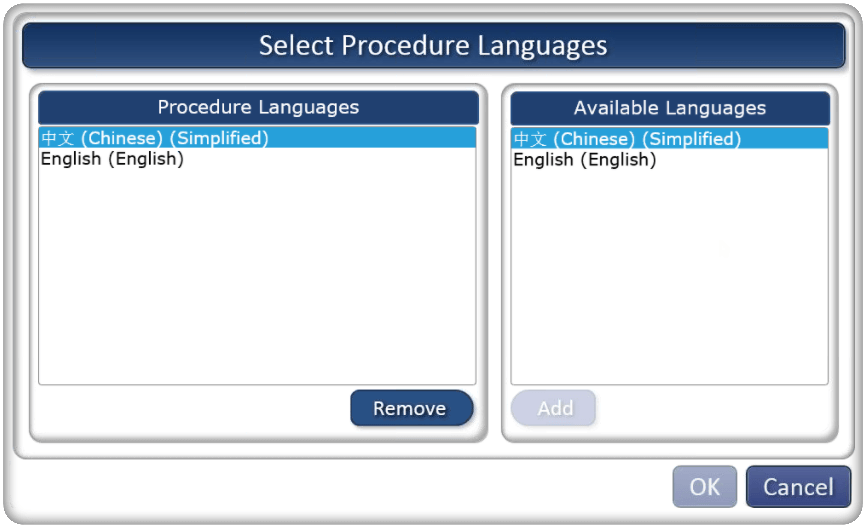
Multilingual SmartProcedures
May 30, 2018
ATR’s next generation of SmartProcedures has architectural changes for multilingual data support. Language identifiers within the data structures allow side-by-side editing and procedure publishing in multiple languages.
2018 Spring Meeting and 15th Global Congress On Process Safety
March 31 - April 4, 2018
The AIChE Spring Meeting, the year’s key technical conference for practicing chemical engineers, covered a wide range of subjects relevant to the current needs of industry.
Elliott Lander’s GCPS paper, Procedure Digitalization: The New Era for Human Factored Procedure Technology, was well received by conference experts in human reliability and performance.
ExxonMobil Team-Building Event
April 28, 2016
ExxonMobil IT (EMIT) sponsored an ATR-ExxonMobil Partnering Happy Hour at The Main Event. Congratulations are due to our entire Tech Team for delivering a 100% build for ExxonMobil. The ExxonMobil team was thrilled and happy about that. Keep up the great work!
Procedure Writer Training and Certification
September 22-25, 2015
High performing facilities recognize the importance of having formal training and certification programs to ensure procedure writers have the necessary skill set to provide highly reliable and technically accurate procedures.
Our writer training and certification includes:
- Procedure attributes, format, and writing methodology
- Procedure Use and Adherence standards
- Overview of the standardized procedure process
- Human factor engineering in procedures
- Findings and recommendations from Texas A&M Advanced Procedure Research Study (ExxonMobil, NOVA Chemicals, Petrofac, NASA and ATR)
- Human error reduction in procedures
- Procedure development and review process
- Procedure review and hands-on exercises
- New technologies (SmartProcedures) accelerating procedure production, quality and consistency
Advanced Procedure Research Study
June 2015
This Texas A&M University study with leading technology provider ATR and a diverse range of industry partners conducts groundbreaking research on leading practices, technology breakthroughs, and human factor engineering to ensure high quality and usable procedures.
Combining leading and future practices, human factors, and technology, this study focuses on improving the quality and usability of procedures to achieve zero incidents. Current practices are limited to "one size fits all" paper-based compliance-driven procedures whereas this study focuses on technology–enabled solutions.
Principal investigators are Dr. Sam Mannan, Director of the Mary Kay O’Connor Process Safety Institute, and Dr. Camille Peres, leading human factors and usability professor at the Health Science Center.